Just what is a "macromolecule"?
A giant molecule that is made up of many small molecules is called a “macromolecule.”
When you think of macromolecules, what comes to mind? A round sphere? Something where the threads entangled with one another? Or, maybe you imagine a difficult chemical formula? ThreeBond R&D staff will explain what macromolecules are and what kind of materials involve “macromolecules.”
First, there are small “molecules”
A “molecule” is a collection of “tiny particles called atoms.” “Hydrogen atoms” do not demonstrate the properties of hydrogen, but “hydrogen molecules” do demonstrate the properties of hydrogen. In this way, the smallest unit that demonstrates properties in terms of the substance involved, is called a “molecule.”
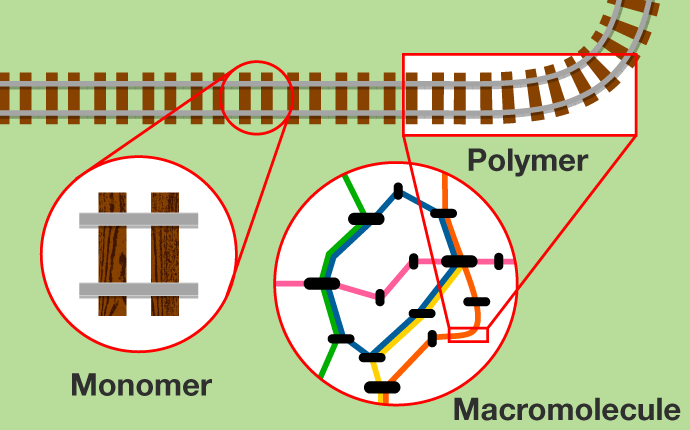
Among these molecules, compounds consisting of relatively small molecules that can be connected repeatedly are called “monomers.” “Polymerization” is the chemical reaction in which many monomers are connected over and over again to form a compound of macromolecules called a “polymer.” In addition, polymers intertwine in complex ways to form huge macromolecule materials such as “cross-linked polymers.”
Let’s make a simple comparison with a train route map. The rails and iron which form the tracks would be monomers, the tracks connect to form a polymer, and the various lines are intertwined to form a huge macromolecule, which ends up looking like the route map we are talking about.
The state changes at the length wherein the molecules are connected
Let’s take polyethylene as an example.
“Polyethylene” is a macromolecule made by repeatedly connecting molecules of “ethylene.” Here, the monomer is ethylene and the polymer is polyethylene.
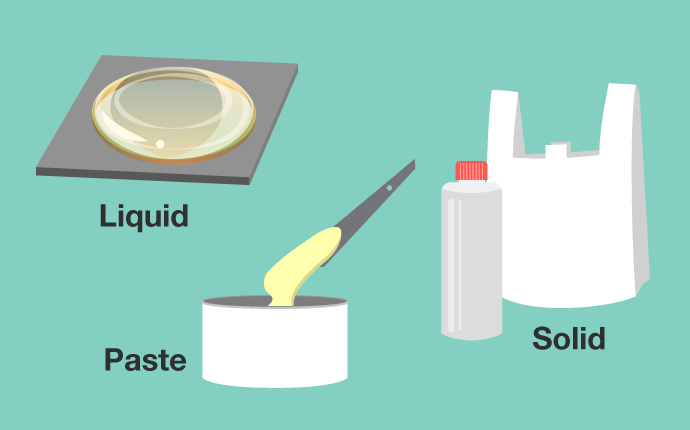
Polyethylene is used in plastic bags, cosmetic containers, and so on. It is a macromolecular material that is familiar to us. The state of polyethylene changes depending on the length of the ethylene connections. As the connection gets longer, they turn into a liquid as is the case with oil, a paste as is the case with wax, and a solid, as is the case with a plastic bag or container.
Even though they are made from the same polyethylene, the “firmness” and “flexibility” found in plastic bags and cosmetic containers differ. This is because the lengths of the connected ethylene differ. Even with the same name, macromolecules see their state and physical properties change when the structure differs.
By the way, the prefix “poly” in the words polymer, polyethylene, and poly bags, comes from Greek and means “many.”
Macromolecules found all around us
There are many macromolecules found all around us. Let’s see what kind of types there are along with where we can find them.
・Natural macromolecules (biological macromolecules): Macromolecules made in the bodies of living things
Examples: natural rubber, meat (protein), plants (polysaccharides), genes, etc.
・Synthetic macromolecules: Macromolecules created by humans
Examples: Synthetic fibers (nylon, vinylon, polyester, polyethylene terephthalate, etc.), synthetic resins (polyvinyl chloride, polyethylene, phenolic resin, etc.), silicone resins (silicone rubber and silicone oil), synthetic rubber, plastic bags, plastic bottles, aquarium tanks (transparent acrylic plates), cosmetic additives, adhesives, etc.
・Inorganic macromolecules: Macromolecules that occur naturally but which are not made in the bodies of living things
Examples: Glass, diamonds, rocks (crystals, quartz, and mica), etc.
・Semi-synthetic macromolecules: Macromolecules that are chemically derived from natural macromolecules
Examples: Celluloid, nitrocellulose, recycled macromolecules (recycled fiber rayon), etc.

By the way, many of the adhesives made by ThreeBond are also classified as “macromolecules.”
Have you now gained an understanding of how “macromolecules” are familiar parts of our lives? Next, we will consider the types and recycling of “plastic,” which is a “synthetic macromolecule” made by humans.
